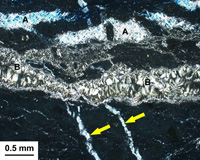
100 year old cement barrels from a shipwreck are an excellent place to study the interaction of seawater with portland cement. The example shown here illustrates the steady progression of chemical reactions that move inward as outer layers spall off. The arrows indicate earlier-formed, gypsum-filled cracks that formed deeper within the barrel. These are now overprinted by brucite (B) and aragonite (A) that form closer to the surface. Brucite forms through reaction with magnesium sulfate in seawater. While an undesirable reaction, the outer brucite layers often armor concrete from further deterioration unless removed by physical attrition.
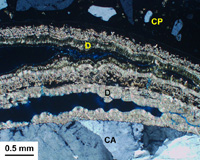
The case shown here represents a concrete foundation for a lighthouse. The concrete was poorly consolidated when placed leaving honeycombs for seawater to permeate. Unlike the example shown above, there is no armoring effect and sulfate reactions have affected a greater depth of the structure. Shown here are layered deposits of brucite and aragonite (D) within air-voids adjacent to a coarse aggregate grain (CA). The cement paste (CP) is thoroughly leached leaving a porous and weakened gelatinous binder composed only of insoluble silica and alumina.